what keeps electrons attracted to an atoms nucleus
Why do electrons non fall into the nucleus?
- Page ID
- 1665
The picture of electrons "orbiting" the nucleus similar planets around the sunday remains an indelible one, non just in pop images of the atom but also in the minds of many of u.s.a. who know meliorate. The proposal, first made in 1913, that the centrifugal forcefulness of the revolving electron just exactly balances the bonny force of the nucleus (in illustration with the centrifugal forcefulness of the moon in its orbit exactly counteracting the pull of the World's gravity) is a nice motion picture, merely is simply untenable.
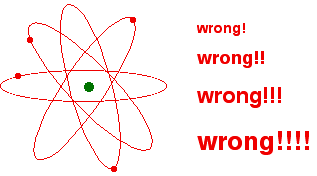
One origin for this hypothesis that suggests this perspective is plausible is the similarity of the gravity and Coulombic interactions. The expression for the strength of gravity between 2 masses (Newton'due south Law of gravity) is
\[F_{gravity} \propto \dfrac{m_1m_2}{r^2}\characterization{one}\]
with
- \(m_1\) and \(m_2\) representing the mass of object i and 2, respectively and
- \(r\) representing the distance between the objects centers
The expression for the Coulomb force between two charged species is
\[F_{Coulomb} \propto \dfrac{q_1q_2}{r^two}\characterization{2}\]
with
- \(q_1\) and \(q_2\) representing the charge of object 1 and 2, respectively and
- \(r\) representing the distance between the objects centers
However, an electron, unlike a planet or a satellite, is electrically charged, and it has been known since the mid-19th century that an electric charge that undergoes acceleration (changes velocity and management) will emit electromagnetic radiation, losing energy in the procedure. A revolving electron would transform the atom into a miniature radio station, the energy output of which would exist at the cost of the potential energy of the electron; co-ordinate to classical mechanics, the electron would simply spiral into the nucleus and the atom would plummet.
Quantum theory to the Rescue!
By the 1920's, it became clear that a tiny object such as the electron cannot be treated equally a classical particle having a definite position and velocity. The best we can practise is specify the probability of its manifesting itself at any point in space. If you had a magic camera that could take a sequence of pictures of the electron in the 1s orbital of a hydrogen atom, and could combine the resulting dots in a unmarried epitome, yous would run into something like this. Clearly, the electron is more than likely to be found the closer nosotros move toward the nucleus.
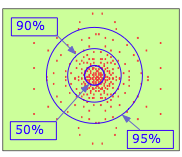
This is confirmed by this plot which shows the quantity of electron accuse per unit of measurement volume of space at various distances from the nucleus. This is known equally a probability density plot. The per unit volume of space role is very important hither; equally we consider radii closer to the nucleus, these volumes get very small, so the number of electrons per unit of measurement volume increases very rapidly. In this view, it appears as if the electron does fall into the nucleus!
According to classical mechanics, the electron would simply screw into the nucleus and the atom would collapse. Quantum mechanics is a different story.
The Boxing of the Infinities Saves the electron from its death spiral
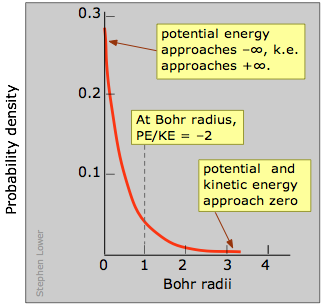
Equally yous know, the potential free energy of an electron becomes more negative as it moves toward the bonny field of the nucleus; in fact, it approaches negative infinity. However, because the full free energy remains constant (a hydrogen atom, sitting peacefully by itself, volition neither lose nor larn energy), the loss in potential energy is compensated for by an increase in the electron's kinetic energy (sometimes referred to in this context as "confinement" energy) which determines its momentum and its effective velocity.
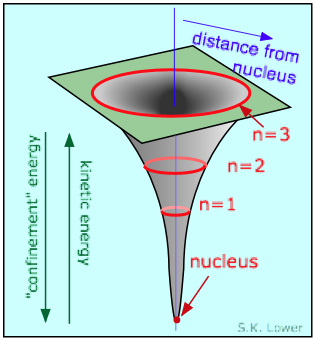
So as the electron approaches the tiny volume of space occupied by the nucleus, its potential energy dives down toward minus-infinity, and its kinetic free energy (momentum and velocity) shoots up toward positive-infinity. This "battle of the infinities" cannot exist won by either side, so a compromise is reached in which theory tells us that the fall in potential free energy is only twice the kinetic energy, and the electron dances at an boilerplate distance that corresponds to the Bohr radius.
At that place is still ane affair incorrect with this motion-picture show; according to the Heisenberg uncertainty principle (a better term would exist "indeterminacy"), a particle equally tiny as the electron cannot be regarded every bit having either a definite location or momentum. The Heisenberg principle says that either the location or the momentum of a breakthrough particle such as the electron can exist known every bit precisely every bit desired, just as one of these quantities is specified more precisely, the value of the other becomes increasingly indeterminate. It is important to understand that this is non simply a matter of observational difficulty, but rather a key property of nature.
What this means is that within the tiny confines of the atom, the electron cannot really be regarded equally a "particle" having a definite free energy and location, so information technology is somewhat misleading to talk about the electron "falling into" the nucleus.
Arthur Eddington, a famous physicist, once suggested, not entirely in jest, that a better clarification of the electron would be "wavicle"!
Probability Density vs. Radial probability
Nosotros can, however, talk most where the electron has the highest probability of manifesting itself— that is, where the maximum negative charge will be found.
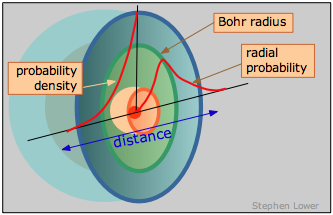
This is just the curve labeled "probability density"; its steep climb as we approach the nucleus shows unambiguously that the electron is most likely to exist found in the tiny book element at the nucleus. Only wait! Did we not only say that this does not happen? What we are forgetting hither is that every bit we move out from the nucleus, the number of these small volume elements situated forth any radius increases very rapidly with \(r\), going up by a factor of \(4πr^two\). So the probability of finding the electron somewhere on a given radius circle is found by multiplying the probability density by \(4πr^ii\). This yields the bend you have probably seen elsewhere, known as the radial probability, that is shown on the right side of the above diagram. The peak of the radial probability for principal quantum number \(n = i\) corresponds to the Bohr radius.
To sum up, the probability density and radial probability plots express two different things: the get-go shows the electron density at any single indicate in the atom, while the second, which is generally more useful to us, tells the states the the relative electron density summed over all points on a circle of given radius.
References
- Why Doesn't the Electron Fall Into the Nucleus? Franklin Bricklayer and Robert Richardson, J Chem. Ed. 1983 (xl-42). See as well the comment on this article by Werner Luck, J Chem Ed 1985 (914).
- For more detailed descriptions of these ii kinds of plots, see this McMaster U. page past Richard Bader.
- The author is grateful to Robert Harrison of U. of Tennessee-Knoxville whose suggestions led to improving this article.
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Why_atoms_do_not_Collapse